GUIDES FOR PATIENTS
Euromed in Numbers
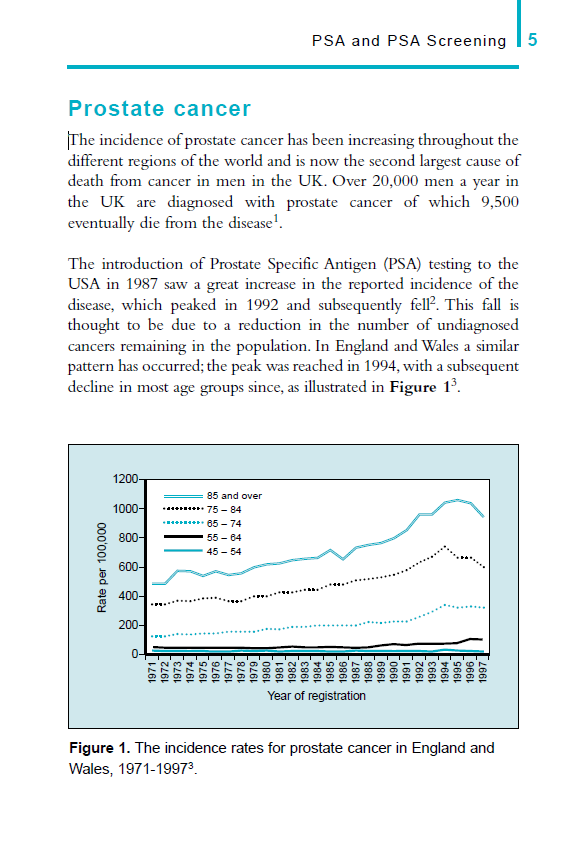
Journal Readership at Feb 2021
Number of Countries we ship to
Publishing since
MEDICAL HANDBOOKS FOR HEALTHCARE PROFESSIONALS
JOURNALS
Euromed Blog
Survey of qualified persons on remote certification
Social distancing posed an interesting question for the Qualified Person (QP) in the early days of the COVID-19 pandemic: Is it permissible, or practically possible, to certify batches for release ...
Techniques for conducting quality audits
Auditors should be direct and avoid asking questions to purposely try and stump the auditee. In addition, auditors should be instructive, explaining why they are requesting the information. Further...
A global study of the performance of cleanroom garments over their life cycle
Sterile garments for cleanroom use often present a variable performance over their entire life cycle as they are vulnerable to damage from laundering and sterilization methods. A study was conducte...
EUROMED SERVICES
E-BOOKS
When you purchase an ebook from us, we will automatically send you any subsequent updated versions of the book to the email you originally purchased the book through.
AUTHORS
We are proud of the relationships we have built over the years with an outstanding and renowned author community. If you would like to contribute a paper to one of our journals or discuss publishing a book, please contact us using the enquries form.
ADVERTISE IN OUR JOURNALS
Let our readers know about your company!; Our Journals go out to a readership of over 150,000 across the globe. The IP is the Journal of the FIP (International Pharmaceutical Federation) and is read by its international membership. Our other journals, the CACR, GMP Review and Pharmacovigilance, are also read by a global audience. To advertise in our of our journals, please contact us for a bespoke package.
MEDICAL STUDENTS
If you are a medical or healthcare student, we offer very special discounts; if your University hasn't already provided you with a discount code, please contact us or ask them to contact us to add your University to our discount scheme.